Lorena Ruíz (UB) — On the Amyloid Beta 40 aggregation and secondary nucleation
Don't miss any Success Story following us on X and LinkedIn!
@RES_HPC RES - Red Española de Supercomputación @res-icts.bsky.social
Check this Success Story at our LinkedIn: On the Amyloid Beta 40 aggregation and secondary nucleation
💡A Success Story about Alzheimer's first stages💡
📋 "On the Amyloid Beta 40 aggregation and secondary nucleation" led by Lorena Ruiz Pérez from Universitat de Barcelona and her team at Institute for Bioengineering of Catalonia (IBEC)
Neurodegenerative diseases caused by Alzheimer's disease (AD) affect over 55 million people and are the 7th leading cause of death globally. It involves an abnormal accumulation of the protein Amyloid Beta (Aβ) in the brain, which forms toxic structures.
Aβ40, the most common form, aggregates into fibrils and oligomers, responsible of disease progression. However, the details of the aggregation and formation of these structures are unclear. To address these issues, the team combined advanced liquid-phase transmission electron microscopy (LPTEM) with molecular dynamics (MD) simulations.
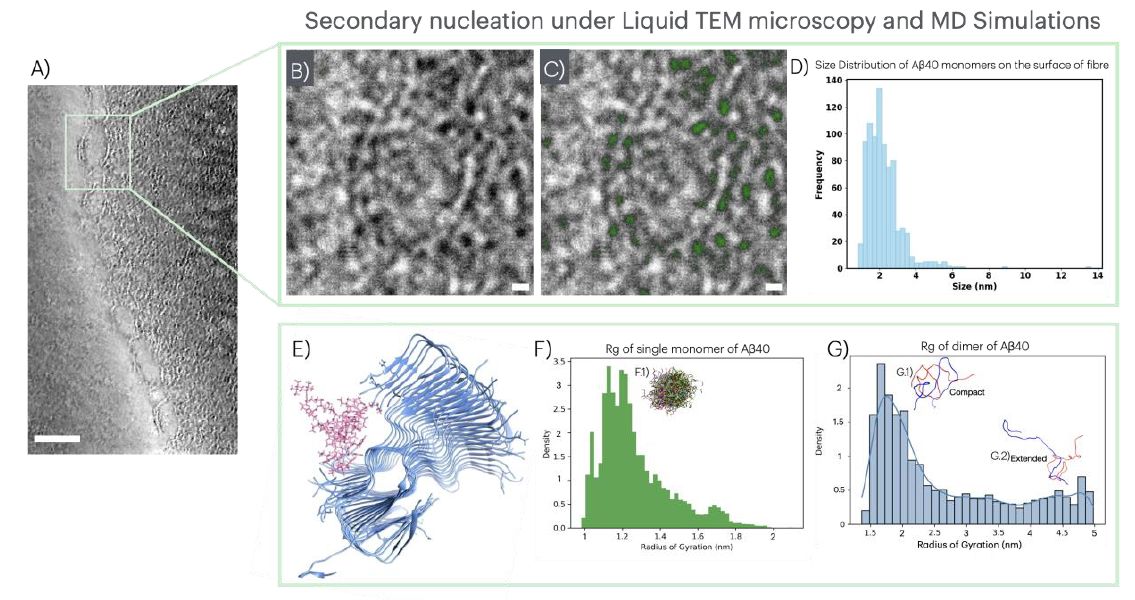
LPTEM allowed to visualize Aβ40 protein monomers attaching to fibrils in real-time, which is the first step for the nucleation of toxic species contributing to cognitive decline.
🖥️ Thanks to RES supercomputer hashtagCibeles from Universidad Autónoma de Madrid, the team simulated the moment these structures form, allowing them to complement and validate microscopy findings.
They found that early signs of their formations occur at the nanosecond scale, far faster than the fastest microscopes and detectors can directly observe. The MD simulations also confirmed what the team saw under the microscope: once a monomer attaches to a fibril surface, it adopts a compact shape.
The particle sizes observed experimentally closely matched values predicted by simulations, specifically the radius of gyration (Rg) that measures how compact or spread is a molecule. Additionally, they identified two primary conformational states for Aβ dimers: "extended" and "compact".
📸 A) Snapshot of a video acquired by LPTEM showing Aβ40 oligomers on the surface of Aβ40 fibrils located at the outer region of a large condensate; (B) Zoomed-in region from (A) showing two fibrils in detail with dark monomers on the fibrils´ surface, and (C) highlighted in green); (D) Size distribution of observed species on fibrils´ surfaces; (E) Final frame of MD simulation illustrating a highly stable, compact monomer attached to a fibril; (F) Radius of gyration Rg for single monomers with (F.1) a cartoon showing the superimposed conformational ensemble from 6000 frames (inset); (G) Rg for dimers, illustrating compact (G.1) and extended (G.2) conformations.